VIRUS TODAY: 3rd vaccine shows promise, death toll soars
Here’s what’s happening on Monday with the coronavirus pandemic in the U.S:
THREE THINGS TO KNOW TODAY
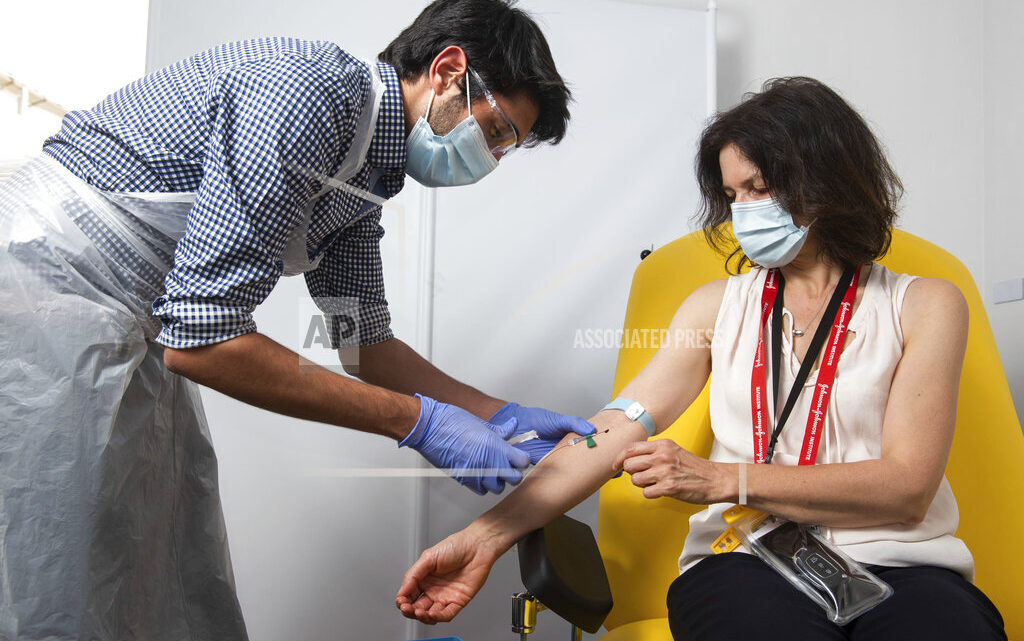
—Pharmaceutical company AstraZeneca said that late-stage trials showed its coronavirus vaccine was up to 90% effective, giving public health officials hope they may soon have access to a vaccine that is cheaper and easier to distribute than some of its rivals. AstraZeneca is the third major drug company to report encouraging news in recent weeks as the world anxiously waits for scientific breakthroughs that will bring an end to a pandemic that has wrought economic devastation and resulted in nearly 1.4 million confirmed deaths.
—Americans are still heading to airports in large numbers to travel for the Thanksgiving holiday, despite the pandemic and guidance from health officials to limit gatherings as the virus rages through the country. More than 1 million people were screened by the Transportation Safety Administration on Sunday — the most on any day since March. Travel numbers are much lower than previous Thanksgiving holidays.
—The switch to remote learning in rural New Mexico has left some students profoundly isolated — cut off from others and the grid by sheer distance. In the village of Cuba, New Mexico, population 800, the school system is sending school buses to students’ homes over an hour away to bring them assignments, meals and a little human contact. On the fringe of the Navajo Nation, many families have no electricity, let alone internet. It is yet another way in which the pandemic has exposed the gap between the haves and have-nots in the U.S.
THE NUMBERS: The U.S. is now averaging more than 1,500 new deaths per day, according to data from Johns Hopkins University. The seven-day rolling average for deaths was less than 1,000 two weeks ago and now is 1,510 There have been close to 1.4 million confirmed deaths globally, and the U.S. has seen the most by far: almost 257,000.
QUOTABLE: “Our goal … to make sure that we have a vaccine that was accessible everywhere, I think we’ve actually managed to do that.” — Dr. Andrew Pollard, chief investigator for the AstraZeneca coronavirus vaccine trial, on the news that the vaccine doesn’t have to be stored at ultra-cold temperatures, making it easier to distribute, especially in developing countries.
ICYMI: A slowdown in industrial activity linked to the coronavirus pandemic has cut emissions of pollutants and heat-trapping greenhouse gases, but hasn’t reduced their record levels in the atmosphere, the United Nations weather agency said on Monday.
ON THE HORIZON: Before any vaccine is permitted in the U.S., it must be reviewed by the Food and Drug Administration, which requires study on thousands of people. Normally, the process to approve a new vaccine can take about a decade. But the federal government is using various methods to dramatically speed up the process.
___
Find AP’s full coverage of the coronavirus pandemic at https://apnews.com/hub/coronavirus-pandemic


No Comment